|
The INTERNATIONAL JOURNAL of APPLIED RESEARCH In Veterinary Medicine |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The International Journal of Applied Research in Veterinary Medicine |
A Cross-sectional
Study of Bovine Tuberculosis in Selected Dairy Farms in Ethiopia
G. Ameni, DVM*
P. Bonnet, DVM, MSc, MBA†
M. Tibbo, DVM‡
*Institute of Pathobiology, Addis Ababa University; home-based at the Faculty of Veterinary Medicine, Addis Ababa University, Addis Ababa, Ethiopia
†International Livestock Research Institute (ILRI), seconded from CIRAD-EMVT, Addis Ababa, Ethiopia
‡International Livestock Research Institute
(ILRI), Animal Genetic Resources, Addis Ababa, Ethiopia
KEY WORDS: Mycobacterium bovis, prevalence, risk factors, zoonosis, Ethiopia
ABSTRACT
A cross-sectional study to determine individual animal prevalence of bovine tuberculosis (BTB) was conducted on 1171 dairy cattle in 12 randomly selected dairy farms in Ethiopia between January 1999 and May 2001 using comparative intradermal tuberculin (CIT) test and bacteriologic study through milk culturing. An overall individual animal prevalence of 46.8% (548 of 1171 animals) and a herd prevalence of 91.7% (11 of 12 farms) were recorded in 12 dairy farms by the CIT test. There were significant (P <0.0001) differences in individual prevalence between farms and breeds (pure Holstein and their crosses with Zebu). There was positive correlation (r = 0.41) between herd size and prevalence of bovine tuberculosis. Furthermore, a negative linear association (R2 = 0.24) was found between mean score of management of the farm and prevalence, indicating that the prevalence of bovine tuberculosis could be improved by sanitary measures. Breed and management affected the prevalence of BTB (R2 = 0.30) as confounding variables. Mycobacterium bovis was isolated from the milk of 13.3% (4 of 30) reactor cows. The widespread occurrence of BTB in the study farms and isolation of M. bovis from the milk of reactor cows signify its economic importance and potential risk to public health. Generalization and improved use of milk pasteurization within all dairy subsectors is recommended, and this would affect the competitiveness of the dairy sector in Ethiopia.
INTRODUCTION
The human population in Ethiopia is estimated at 67 million and is growing by approximately 3.5% per year. Tegegne and Gebrewold1 reported that this figure would increase to approximately 139 million by the year 2020, making Ethiopia the third most populous country in Africa. The number of children under the age of 15 is projected to increase from 26.7 million in 1994 to 59.0 million in 2020. As a result, the demand for animal products, in terms of both quantity and quality, is estimated to increase substantially, thereby requiring intensification of animal production integrated with proper feeding, animal health management, and genetic improvement. Additionally, potential domestic dairy market within growing urban demand2,3 and export potential of animal and animal products are great, but to this effect, control of diseases such as bovine tuberculosis (BTB) and other diseases is a prerequisite.4
BTB is an infectious disease of cattle caused by Mycobacterium bovis and is characterized by the formation of tubercles in any tissue/organ of the animal. It is zoonotic, being transmitted to humans by an aerogenous route and/or through consumption of infected milk and other cattle products. The World Health Organization estimates human tuberculosis (TB) incidence and death for 1990–1999 to be 88 million and 30 million, respectively, with most cases in developing countries.5 Zoonotic TB is present in animals in many developing countries where surveillance and control activities are often inadequate or unavailable.6 Ethiopia is one of these countries, and many epidemiologic and public health aspects of the infection remain largely unknown. This study was formulated to generate baseline information on the prevalence of BTB and related risk factors in selected dairy farms in Ethiopia.
MATERIALS AND METHODS
Study Subjects and Sampling
The study was conducted on 1171 dairy cattle belonging
to 12 randomly selected dairy farms. All animals whose age was more
than 6 months were included. Approximately 67% of the study farms were
situated closer to Addis Ababa and hence, were milk sheds for the city,
the capital of Ethiopia. The rest of the farms were milk sheds for major
towns in central Ethiopia: Ambo College and Gadissa farms are located
at Ambo (the town of West Shoa Zone); Chaffa farm is found in South
Wollo and serves for Dessie, Kombolcha, and Kemissie; and Alagae farm
serves for Zeway and Shashemene towns.
Management of Farms
The management of the farm was scored on the basis of degree of ventilation, neatness of the floor, degree of exposure to the sunlight, stocking rate, neatness of watering and feeding. The score of each of these variables ranged from 1 to 3 (ordinal scoring established from an expert viewpoint), the former being the poorest score whereas the latter being the best score. These variables were scored for each farm. Mean score of the management parameters was used to measure the association related to the prevalence of bovine tuberculosis.
Comparative Intradermal Tuberculin Test
Two sites 10 cm apart on the midneck of an animal were shaved and the skin thickness was measured (in millimeters) with calipers before the injection of tuberculin. Aliquot of 0.1 mL of 20,000 IU/mL bovine PPD (Bovituber; Rhône Mérieux, France) and 0.1 mL of 25,000 IU/mL avian PPD (Avituber; Rhône Mérieux) were injected into the dermis of these sites. After 72 hours, the thickness of the skin at the sites was measured again and interpreted according to Office International des Epizooties.7
Milk Culturing
Approximately 30 mL of milk was drawn from the 4 quarters of each the 30 cows that were positive to the comparative intradermal tuberculin (CIT test) under aseptic conditions at the end of milking. The samples were centrifuged at 3000 rpm for 10 minutes at room temperature. The supernatant was decanted and the sediment was decontaminated with 2% NaOH, centrifuged again, and neutralized with H2SO4. After neutralization, each sediment was inoculated onto 2 Lowenstein-Jensen media (one with pyruvate and the other without pyruvate). The culture was incubated at 37˚C and 5% CO2. Observation for bacterial growth was made for 12 weeks. The growth of white, moist, flat and nonpigmented friable colonies on the pyruvate-enriched medium were considered as the primary cultures of M. bovis.
Statistical Analysis
The animal prevalence was the proportion of CIT-positive animals out of the total number of animals tested. This was generated by FREQ procedures of the Statistical Analysis System.8 Herd status was considered positive when at least one animal showed a positive test, and herd prevalence was calculated afterward. Variation of prevalence between farms and breeds was investigated by the chi-squared test. Pearson’s correlation coefficients were used to estimate the relationships of the within-herd prevalence of bovine tuberculosis with herd size. Relationships of the within-herd prevalence of bovine tuberculosis with mean management score and breed (coded as 1 for pure and 2 for crosses) were obtained by regressing prevalence on these factors.
RESULT
Prevalence
Table 1 presents the individual animal prevalence of each of the 12 farms. An overall animal prevalence of 46.8% (548 of 1171 animals) and a herd prevalence of 91.7% (11 of 12 farms) were recorded in 12 dairy farms by the CIT test. There was significant difference in animal prevalence between farms (c2 = 333.75; P <0.0001). There was also significant (c2 = 65.95; P <0.0001) difference in animal prevalence between pure Holstein and their crosses with Zebu. There was a positive correlation (r = 0.41) between herd size and prevalence of bovine tuberculosis. Furthermore, a negative linear association (R2 = 0.24) was found between mean score of management of the farm and animal prevalence, indicating that the prevalence of bovine tuberculosis could be improved by sanitary measures (Fig. 1). Breed and management affected the animal prevalence of BTB (prevalence = 102.92–17.95 breed –20.80 mean management score; R2 = 0.30) as confounding variables.
Isolation From Milk
M. bovis was isolated from the milk of 13.3% (4 of 30) reactor cows on the basis of growth characteristics and niacin test according to OIE (1992).
DISCUSSION
The overall prevalence recorded by the present study was high as compared with previous results.9,10 This is because there has never been control measures for BTB in Ethiopia. Hence, even if one animal is infected in such a farm with a large herd size, there is a high chance of transmitting the disease to other members of the herd. Additionally, given the scarce resource on dairy breeds in Ethiopia, there is a tendency to keep animals with a long production life without culling, reinforcing the chance that they participate in BTB spread. The high prevalence recorded in some farms has serious implications and thus calls for attention of the concerned sectors.
In the present study, the animal prevalence of BTB varied among the 12 farms and significantly differed between the herd size and breed. The animal prevalence of BTB has increased with increased herd size. This could be the result of the fact that in the farms with smaller herd size, the lower number of animals limits the chances of transmission, because the transmission rate of BTB is dependent on herd size.11 In addition to herd size, management of the farm was an important factor that affected the animal prevalence of BTB. Exceptionally low BTB prevalence with a herd size of 205 animals was recorded in the Alagae farm (Table 1). This could be associated with the best management of this farm compared with other farms: no confinement, separate watering and feeding troughs, optimal stocking rate, and animals move out of their barn regularly for exercise and sun bath. O’Reilly and Daborn11 and Kiros12 reported higher prevalence of BTB in animals kept under poor management than animals in good management. A poor management system promotes the chance of transmission of infection and development of the disease.13 The difference in prevalence between pure Holstein and their crosses with zebu was significant. Similar results were reported from India,14 Turkey,15 and Ethiopia.10 Barwinek and Taylor15 reported that genetically improved cattle might suffer more severely from malnutrition and poor housing systems in tropical countries, and consequently become more susceptible to infection.
M. bovis was
isolated only from the milk of 4 (13.3%) of the 30 CIT-positive cows.
This substantiates the fact that TB in cattle is principally a pulmonary
disease; only approximately 1% of the tuberculous cows excrete tubercle
bacilli in their milk.16 This shows that cows most probably transmit
the disease by the aerogenous route.17 Collins and Grange18 indicated
that as TB spreads among cattle primarily by the aerogenous route, those
working with cattle are more likely to develop pulmonary disease than
the alimentary type of the disease. According to these workers, the
apparent decline in the incidence of BTB in humans is the result of
underreporting, because few clinical laboratories distinguish between
the bovine strain and human strain. Under most circumstances, approximately
90% tuberculosis infections in cattle occur by the respiratory route.19
In Britain, it was
reported that the pathologic examination of cattle over 6 months old
showed that the primary complex is in the lungs and their associated
lymph nodes in at least 60% of cases.20 Different workers have reported
that pulmonary tuberculosis resulting from M. bovis is more common among rural than urban dwellers.21
Schmiedel22 reported that compulsory pasteurization of milk had little
impact on the incidence of pulmonary tuberculosis resulting from M. bovis in
rural population, whereas it markedly reduced the incidence of abdominal
tuberculosis resulting from this bacillus in town dwellers.
The
present study showed the widespread occurrence of BTB in cattle in the
study farms. Furthermore, the isolation of M. bovis from the milk of reactor
cows signifies the actual and potential risks of BTB to human beings.
Although the number of M. bovis-positive milk samples
was low, pooling milk from these farms does pose a great public health
danger to milk consumers as Kleeberg23 has shown that one cow can excrete
enough viable bacilli to contaminate the milk of up to 100 cows when
their milk is pooled, which was the case in the governmental dairy collection
system.2 Therefore, disease surveillance program should be initiated
as a priority. To this effect, intersectoral collaboration between the
smallholder farmers, medical and veterinary professionals should be
established to evaluate the scale of the problem.
ACKNOWLEDGMENTS
The authors thank the Research and Publication Office of the Addis Ababa University for financial support. The service rendered from the TB laboratory of the Armauer Hansen Research Institute (AHRI) is highly appreciated. Technical support of Haimanot Gebreexabher (AHRI), Hailu Getu, Nega Nigussie, and Demeku Nega is highly appreciated.
REFERENCES
1. Tegegne A, Gebrewold A: Prospects for peri-urban development in Ethiopia. In: Proceedings of the 5th Annual Conference of the Ethiopian Society of Animal Production (ESAP), held in Addis Ababa, ESAP, Addis Ababa, Ethiopia, 1998:28–39.
2. Hurrisa B, Mahmud A, Teferi HL, Lemma A: Dairy Products Marketing Survey in Addis Ababa and Surrounding Regions. Addis Ababa: Dairy Development Enterprise, 1994:84.
3. Bonnet P, Duteurtre G: Diagnostic et Dynamique de la filière laitière bovine du bassin d’approvisionnement de la ville d’Addis Abeba (Ethiopie–Afrique de l’Est). Bilan sur les composantes périurbaine et urbaine. Agriculture périurbaine en Afrique subsaharienne, Colloque Avril 1997. Montpellier, France: CIRAD, CORAF, 1997.
4.Bonnet P: Des recommandations pour le développement de bassins laitiers en Afrique. Afriq Agric 286:59–60, 2000.
5. Report of WHO Meeting on Zoonotic Tuberculosis (Mycobacterium bovis) With the Participation of FAO. Geneva: World Health Organization, 1993:1–26.
6. Cosivi O, Grange JM, Daborn CJ, et al: Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg Infect Dis 4:1–17, 1998.
7. OIE: Bovine Tuberculosis. OIE Manual for Diagnostic Techniques of Livestock Diseases. Paris: OIE, 1992:287–296.
8. SAS: SAS User’s Guide for Personal Computers, version 8. Under Windows 2000, Cary, NC: SAS Institute. 1998.
9. Assegid B, Lübke-Becker A, Lemma E, Taddele K, Britton S: Bovine tuberculosis: a cross sectional and epidemiological study in and around Addis Ababa. Bull Anim Hlth Prod Afr 48:71–80, 2000.
10. Ameni G, Ragassa A, Kassa T, Medhin G: Survey on bovine tuberculosis and its public health implications to cattle raising families in Wolaita Soddo, Southern Ethiopia. Ethiop J Anim Prod 1:55–62, 2001.
11. O’Reilly LM, Daborn CJ: The epidemiology of Mycobacterium bovis infection in animals and man: a review. Tubercul Lung Dis 1:1–46, 1995.
12. Kiros T: Epidemiology and zoonotic importance of bovine tuberculosis in selected sites of Eastern Shoa, Ethiopia. MSc Thesis, Faculty of Veterinary Medicine, Addis Ababa University and Free University of Berlin, 1998.
13 Morris RS, Pfeiffer DU, Jackson R: The epidemiology of mycobacterium infections. Vet Microbiol 40:153–177, 1994.
14. Ram T, Sharma RM: Tuberculosis infection in Harayana and Hissar cattle. Indian J Vet Sci Anim Husband 25:99–104, 1965.
15. Barwinek F, Taylor NM: Assessment of Socio-economic Importance Bovine Tuberculosis in Turkey and Possible Strategies for Control or Eradication. Ankara: Turkish-German Health Information Project, General Directorate of Protection and Control, 1996:3–45.
16. Pritchard DG: Century of bovine tuberculosis 1888–1988: consequences and controversy. J Comp Pathol 99:357–399, 1988.
17. Grange JM, Yates MD: Zoonotic aspects of Mycobacterium bovis infection. Vet Microbiol 40:137–151, 1994.
18. Collins CH, Grange JM: Zoonotic implications of Mycobacterium bovis infection. Ire Vet J 41:363–366, 1987.
19. Francis J: Tuberculosis in Animals and Man. A Study in Comparative Pathology. London: Cassell, 1958:16.
20. Stamp JT:
A review of the pathogenesis and pathology of bovine tuberculosis with
special reference to practical problems. Vet Rec 56:443–446, 1944.
21. Mangus K: Epidemiological basis of tuberculosis eradication. Risk of pulmonary tuberculosis after human and bovine infection. Bull WHO 36:483–508, 1966.
22. Schmiedel A: A rapid decline in human tuberculosis and persistence of widespread tuberculosis of cattle. Unusual epidemiologic situation and its consequences. Bull Int Tubercul 41:297–300, 1968.
23. Kleeberg HH: Human tuberculosis of bovine origin
in relation to public health. Rev Sci Tech Off Int Epiz 3:11–32,
1984.
Figure.
1. Linear relationship between prevalence of bovine tuberculosis
and mean management score (1 = poor; 3 = best) of the farms
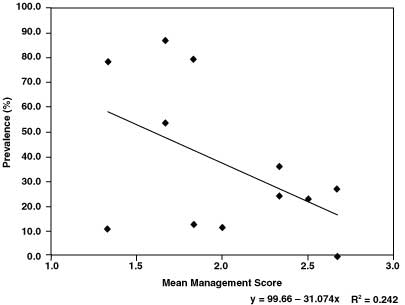
Table 1.
Effect of Beed and Farm on Individual
Animal Prevalence of Bovine Tuberculosis as Assessed by Comparative
Intradermal Tuberculin Test
Herd Size Doubtful Positive Prevalence Chi-square
Farm 333.8*
Debre Zeit Research Centre 78 10 18 23.08
Debre Zeit State Farm 140 1 122 87.14
Debre Zeit Military Camp 52 2 41 78.85
Alagae Farm (Zeway) 205 26 56 27.32
Cheshire Home (Holleta) 70 13 17 24.29
Ambo College (Ambo) 89 20 32 35.96
Chaffa Farm (Wollo) 112 5 89 79.46
Mullo State Farm 300 16 161 53.67
Gadissa Farm (Ambo) 44 4 5 11.36
Tegegn Farm (Sebeta) 37 8 4 10.81
Alemayehu Farm (Selalle) 21 7 0 0.00
Asrat Farm (Selalle) 23 5 3 13.04
Breed 66.0*
Cross (Holstein X Zebu) 232 41 55 23.71
Pure Holstein 936 76 493 52.50
*Chi-square: P <0.0001.
ISSN# 1542-2666