|
The INTERNATIONAL JOURNAL of APPLIED RESEARCH In Veterinary Medicine |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The International Journal of Applied Research in Veterinary Medicine |
The Effect of a
Hemoglobin-Based Oxygen Carrier on Acceptance and Lipid Peroxidation
of Free Skin Grafts in Rats
James P. Farese, DVM, DACVS
David Carmona, DVM
Kathleen Collins, DVM, DACVS
Carol J. Detrisac, DVM, PhD
James Van Gilder, BS
Donald Armstrong, PhD
Department of Small Animal Clinical Sciences
College of Veterinary Medicine, University of Florida
Gainesville, Florida, USA
Funding for this study was provided through a grant
from the American College of Veterinary Surgeons and the Mark Bloomberg
Resident Scholarship Fund.
KEY WORDS: Hemoglobin-based oxygen carrier, rats, skin grafts, hyperbaric oxygen.
Abstract
Hemoglobin-based oxygen carriers (HBOCs) may have the potential to enhance survival of free skin grafts by improving oxygen delivery to the grafted tissue. However, enhanced oxygen delivery to hypoxic graft tissue may also promote the formation of oxygen-derived free radicals and cellular lipid peroxidation, worsening reperfusion injury. Twelve male Sprague-Dawley rats were divided into a control group (n = 6) and a treatment group (n = 6). The control group received 10 mL/kg sterile saline via an intraosseous route immediately after surgery and again on days 3 and 6, and the treatment group received 10 mL/kg Oxyglobin® via an intraosseous route after the surgical procedure and again on days 3 and 6. TBAR analysis of skin samples obtained on days 1, 3, 5, and 7 postoperatively did not reveal differences between groups. Similarly, no obvious visual difference was seen in graft viability between groups. Oxyglobin® does not appear to affect the acceptance of free skin grafts in rats, nor does it appear to exacerbate the production of oxygen-derived free radicals and lipid peroxidation.
Introduction
Free skin grafts are used commonly to treat wounds resulting from degloving injuries and extensive burns.1 However, survival of grafted skin is not always complete, especially when full thickness grafts are used. Survival of the graft at the recipient site is dependent upon plasmatic imbibition and development of new blood supply from the graft bed.2 Newly grafted skin undergoes an initial period of hypoxia from the vascular compromise caused by surgery. During the first 48 to 72 hours after surgery, oxygenation of the graft is accomplished via incorporation of serum containing erythrocytes and leukocytes.
The process of free skin grafting necessitates that the graft become transiently ischemic and hypoxic. When oxygen is reintroduced into the hypoxic tissue during reperfusion, oxygen-derived free radicals are produced and products of cellular lipid peroxidation accumulate.3 This process of reperfusion injury may negatively impact the survival of free grafts.4 Previous studies in free skin grafts in rats have shown that the maximal rate of accumulation of oxygen-derived free radicals attributable to postischemic reperfusion corresponded with the known time of graft recirculation and revascularization (approximately 4 days after surgery).3,4
Hyperbaric oxygen (HBO) therapy has been shown to transiently increase tissue oxygen tension in grafts and improve graft survival in human and animal patients.5-7 This is thought to be caused by acceleration of revascularization. Although hyperbaric oxygen therapy is minimally invasive, it is not widely used because hyperbaric chambers are not readily accessible and cost of treatment is prohibitive. Furthernore, researchers have also suggested that free skin grafts treated with hyperbaric oxygen are at greater risk for reperfusion injury, resulting in lipid peroxidation, than are free skin grafts without HBO treatment.4
Oxyglobin® (Biopure Corporation, Cambridge, MA, USA) contains polymerized hemoglobin of bovine origin in an isotonic solution. It is indicated for the treatment of anemia in dogs. Hemoglobin-based oxygen carriers (HBOC) such as Oxyglobin® increase plasma hemoglobin concentration and oxygen content. When compared with hemoglobin, Oxyglobin® more readily unloads oxygen at the tissue level. This happens because of a decreased affinity for oxygen, causing a right shift in the oxygen-hemoglobin curve. HBOCs have been shown to reduce tumor hypoxia and thereby enhance the effect of radiation therapy.8 They have also been shown to improve tissue oxygenation in striated skin muscle undergoing ischemic injury and protect tissue from reperfusion injury.9
Several reports of using agents to enhance the survival of free skin grafts have been published;10,11 however, no reports of using HBOCs have been seen. HBOCs have the potential to enhance survival of free skin grafts by improving oxygen delivery to the grafted tissue. However, enhanced oxygen delivery to hypoxic graft tissue may also promote the formation of oxygen-derived free radicals and cellular lipid peroxidation (ie, reperfusion injury). Such free-radical damage could compromise graft acceptance. The objectives of this study were 1) to characterize the effect of systemic treatment with Oxyglobin® on free skin graft acceptance and 2) to determine whether systemic administration of Oxyglobin® alters the level of lipid peroxidation within graft tissue.
Materials and Methods
The study was divided
into 2 parts. Part I was designed to characterize the effect of Oxyglobin®
on free skin graft acceptance subjectively and histologically, and
Part II was designed to evaluate the effect of Oxyglobin® on lipid
peroxidation within grafted skin. For both portions of the study,
Sprague-Dawley rats were used in accordance with the guidelines established
by the Institutional Animal Care and Use Committee, and the anesthetic
and surgical protocols were identical. Before the surgical procedure,
all rats were acclimatized for 5 days. The rats were housed individually
and were allowed unlimited access to food and water.
For all rats, anesthesia was induced via intraperitoneal administration of a combination of ketamine (100 mg/mL) and xylazine (8 mg/mL) at a dosage of 1 mL/kg.15 After an acceptable plane of anesthesia was reached, a 20-gauge intraosseous catheter was placed in the right intertrochanteric fossa of the femur of each rat. The hair over the thorax of each rat was clipped, and the skin was surgically prepared with povidone-iodine surgical scrub. A 3 ¥ 3 cm area of skin on the left hemithorax was marked with a sterile marking pen and incised. The skin was completely elevated from the thorax, and subcutaneous tissues were subsequently removed. The graft was then rotated 90˚ in a clockwise fashion and replaced over the exposed panniculus carnosum muscle in the graft bed. The graft and skin edges of the wound bed were apposed by simple interrupted sutures using 4-0 nylon. The thorax of the rats was then bandaged with a nonadherent dressing and 2-inch cotton roll gauze to protect the graft site. For postoperative analgesia, buprenorphine (0.001 mg/kg) was administered subcutaneously immediately after the surgical procedure and again every 8 hours for the next 24 hours. The bandage was removed and replaced every 3 days until the end of the treatment period.
Part I: Assessment of graft acceptance: Twenty-three male Sprague-Dawley rats were divided into a control group (n = 12) and a treatment group (n = 11). The control group received 10 mL/kg sterile saline via an intraosseous route immediately after surgery and again on days 3, 6, and 9. Rats in the treatment group received 10 mL/kg Oxyglobin® intraosseously immediately after the surgical procedure and again on days 3, 6, and 9. The timing of Oxyglobin® administration was chosen based on its clearance time from plasma at this dosage (4 to 5 days). At the end of the study (day 10), graft viability was assessed by visual inspection and histopathologic examination. In addition, a photograph of each graft was taken to document the appearance of the graft on day 10. Grafts that were firm and black or white and liquefactive were considered necrotic and nonviable. Grafts in which the dermis was adhered to the panniculus carnosum muscle were considered viable. All rats were euthanatized via intraperitoneal injection of sodium pentobarbital (150 mg/kg) on day 10. After graft assessment, the entire graft and a margin of normal tissue were harvested and placed in 10% buffered formalin.
The tissue section that was used for histologic assessment of viability for each rat was created by sectioning the graft in a cranial to caudal direction. This was done such that the section contained the most viable (as assessed by gross inspection) portion of the graft (ie, area of greatest take), the cranial and caudal graft edges, and a small amount of the normal adjacent skin. Five-micron sections were stained with hematoxylin and eosin. Engraftment was subjectively assessed by characterizing the total area of contact and vessel ingrowth between the graft and the panniculus carnosum muscle. Total graft failure was indicated by full thickness necrosis of the overlying graft (donor tissue) along with extensive neutrophilic infiltration.
Part II: Assessment of Lipid Peroxidation: Twelve male Sprague-Dawley rats were divided into a control group (n = 6) and a treatment group (n = 6). The control group received 10 mL/kg sterile saline intraosseously immediately after surgery and again on days 3 and 6. Rats in the treatment group received 10 mL/kg Oxyglobin® intraosseously immediately after the surgical procedure and again on days 3 and 6. On days 1, 3, 5, and 7, a 4-mm biopsy punch was used to collect skin samples from each of the four graft quadrants for TBAR analysis. Immediately after, each biopsy sample was frozen in liquid nitrogen and then stored at -80˚C until processing.
Thiobarbituric acid-reactive substance (TBARS) levels were measured in all biopsy samples. Tissue samples were minced with a razor blade and homogenized using a Teflon pestle in a glass tube in 1 mL of buffer solution. A modification of the Yagi method was used for the TBAR measurement.12,13
Data Analysis: TBARS levels were compared between the two groups on each of the biopsy days with a t-test or Mann-Whitney U test when the normaility test failed. Significance was set at P < 0.05.
Results
Part I: Gross appearance of the grafts varied from 100% viability to isolated areas necrosis. Histologic inspection of the grafts revealed varying degrees of epidermal and dermal necrosis, hair shaft necrosis, epidermal hyperplasia, muscle regeneration, neutrophilic inflammation, and seroma. Seromas were present to some degree in all rats and ranged from 10% of the length of the histologic section to full length. Despite the occurrence of seromas, all grafts were at least partially viable. Subjectively, no gross or histologic visual differences were noted between the two groups.
Part II: Mean TBARS concentrations for Oxyglobin®-treated and saline-treated groups for each day of biopsy sampling are shown in Figure 1. A similar pattern of change in TBARS was seen in the two groups with levels increasing after day 5. Differences between groups were not significant on any of the days.
Discussion
In the present study, systemic treatment with Oxyglobin® during the postoperative period did not appear to compromise graft acceptance nor exacerbate reperfusion injury. This is in contrast to the effect of HBO, which has been reported to significantly enhance lipid peroxidation in grafted skin.4 Because the extent of graft survival in the control group was so high, the ability of Oxyglobin® treatment to enhance free skin graft survival postoperatively is still unknown. To determine this, a model that uses a compromised skin graft would need to be used. For example, Lees et al.14 showed that to see the beneficial effect of topical basic fibroblast growth factor (bFGF), grafts must first be compromised in a certain way. In this study, cryoinjury of the graft was induced before grafting by submerging the grafts in liquid nitrogen. Our findings suggest that treatment of free skin grafts with Oxyglobin® does not appear to increase the likelihood of graft failure or worsen lipid peroxidation within the grafted tissue.
No significant difference in lipid peroxidation (as measured by TBARS) was found on any of the days evaluated between the control and treatment groups. Interestingly, TBARS levels for the Oxyglobin® treatment group were significantly higher on day 7 than on days 1 and 3, and although not statistically significant, the same trend was seen within the control group. The timing of this increase in the Oxyglobin® treated rats is difficult to explain given the findings of a previous study performed by McCarthy et al.,3 in which the maximal rate of accumulation of products of lipid peroxidation in nontreated free skin grafts in the same experimental model was between days 3 and 4. In the present study, TBARS levels did not start to increase until after day 5. This discrepancy may be attributed to the fact that the TBARS analysis in this experiment was done via a fluorometric method as opposed to the colorimetric method used in the study by McCarthy et. al. The fluorometric assay is more specific for lipid peroxidation products and fewer problems with absorption of other compounds are found at the same wavelength.
Interestingly, seroma formation did not appear to affect graft survival, because some grafts had full length seromas and were viable. This was an unexpected finding because seroma formation is a known risk factor for free skin graft failure.2 It was evident during histologic evaluation of rats with full-length seromas that vessel ingrowth occurred only from the perimeter of the wound. Thus, it appears that this adjacent vessel ingrowth is adequate enough to maintain the graft tissue, at least in 3 ¥ 3 cm free grafts in rats. This observation was consistent in both groups.
Initially, we attempted to use tail vein catheters to administer the Oxyglobin®; however, this method proved to be unreliable and the catheters were difficult to maintain. As an alternative, we elected to use intraosseous catheters and found them to be easier to place and more reliable. To our knowledge this is the first report of intraosseous catheterization of rats in the literature. This route was well tolerated by the rats although daily administration of Oxyglobin® or lactated ringers solution was usually time consuming due to slow injection rates caused by resistance via this route.
In conclusion, Oxyglobin®
does not appear to affect the acceptance of free skin grafts in rats,
nor does it appear to exacerbate the production of oxygen-derived
free radicals and lipid peroxidation. Whether Oxyglobin® can enhance
free skin graft survival is unknown. Future studies should evaluate
the effect of Oxyglobin® on compromised free skin grafts as well as
ischemic skin flaps. Such studies would help to determine whether
HBOCs have the potential to be used beneficially in skin reconstructive
surgery.
References
1. Swaim SF, Henderson RA: Wound healing. In: Small animal wound management. 2nd Ed. Baltimore: Williams & Wilkins; 1-8, 1997
2. Pavletic MM: Free Grafts. In: Atlas of Small Animal Reconstructive Surgery. 2nd Ed. Philadelphia: W.B. Saunders; 276-279, 1999,
3. McCarthy PE, Hosgood G, Church DF: Lipid peroxidation in free skin grafts in rats. Am J Vet Res 57:216-219, 1996.
4. Lemarie RJ, Hosgood G, VanSteenhouse J, et al: Effects of hyperbaric oxygen on lipid peroxidation in free skin grafts in rats. Am J Vet Res 59:913-917, 1998.
5. Ketchum SA, Thomas AN, Hall AD: Angiographic studies of the effect of hyperbaric oxygen on burn wound revascularization. In: Wada J, Iwa T (eds): Proceedings of The Fourth International Congress of Hyperbaric Medicine. New York: Igaku Shion Medical Publishers; 388-394, 1970.
6. Perins DJD, Cantab MB: Influence of hyperbaric oxygen on the survival of split skin grafts. Lancet 868-871, 1967.
7. Hosgood AD, Elkins AD, Hill RK: Hyperbaric oxygen therapy: Mechanisms and potential applications. Comp Cont Ed Pract Vet 12:1589-1593 1990.
8. Teicher BA, Dupuis NP, Emi Y, et al: Increased efficacy of chemo- and radio-therpay by a hemoglobin solution in the 9L gliosarcoma. In vivo 9:11-18, 1995.
9. Nolte D, Pickelmann S, Swaid S, et al: Oxygen-carrying solutions improve tissue oxygenation in striated skin muscle subjected to critical ischemia. Mund Kiefer Gesichtschir 7:31-35, 2003.
10. Hosgood G, Hodgin C, Strain GM, et al: Effect of deferoximine and hyperbaric oxygen on free, autogenous, full-thickness skin grafts in dogs. Am J Vet Res 56:241-246, 1995.
11. Hosgood G., Lewis DD, Hodgin C, et al: Effect of deferoxaminine-hydroxyethyl pentefraction starch on free, autogenously full-thickness skin grafts in dogs. Am J Vet Res 54:341-347, 1993.
12. Armstrong
D, Browne R: The analysis of antioxidant enzymes and compounds related
to oxidative stress as applied to the clinical chemistry laboratory.
Adv Exp Biol 366:43-58, 1994.
13. Yagi K: A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med 15:212-216, 1976.
14. Lees VC, Fan TP: A freeze-injured skin graft model
for the quantitative study of basic fibroblast growth factor and other
promoters of angiogenesis in wound healing. Br J Plast Surg 47:349-59,
1974.
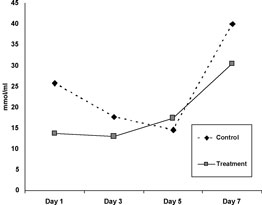
Figure
1. Mean TBARS levels (nmol/mL)
in control and treated (systemic Oxyglobin®) grafts over a 7-day period.
No significant difference was seen between control and treated samples
on any days
ISSN# 1542-2666