|
The INTERNATIONAL JOURNAL of APPLIED RESEARCH In Veterinary Medicine |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The International Journal of Applied Research in Veterinary Medicine |
The Ability of Fipronil
To Prevent Transmission of Borrelia burgdorferi, the
Causative Agent of Lyme Disease To Dogs
Richard Jacobson, PhD†
John McCall, PhD‡
James Hunter III, PhD§
Roberto Alva, DVM, MS, PhD§
Jennifer Irwin, BS§
Andrew Eschner§
Philippe Jeannin, DVM, MS§
Albert Boeckh, DVM, PhD, DACVCP§
†Cornell University, College of Veterinary Medicine, USA
‡University of Georgia, College of Veterinary Medicine, USA
§Merial USA and Merial France
KEY WORDS: Borrelia, ixodes, disease transmission, lyme disease, dog
Abstract
Objective: To assess the efficacy of fipronil spray and fipronil/(S)-methoprene spot-on against Ixodes scapularis and in preventing tick transmission of Borrelia burgdorferi.
Design: Replicated, blinded randomized, controlled study.
Animals: Forty-eight beagle dogs.
Procedure: Dogs
were matched by body weight and randomly allocated, 8 per group, to
untreated controls or to treatment with fipronil spray (2 groups)
or fipronil/(S)-methoprene spot on (2 groups), applied either 7 or
28 days before challenge with B. burgdorferi-infected ticks. Tick
counts were conducted 48 and 120 hours after challenge. Assessment
of successful B. burgdorferi transmission was performed using serology
and skin biopsies from tick attachment sites for culture and polymerase
chain reaction assay.
Results: I. scapularis infestation and B. burgdorferi transmission was successful in all control dogs. Fipronil spray prevented all dogs from becoming infected with B. burgdorferi and was 100% and 99.6% effective against tick infestation 48 hours after challenge in dogs treated 7 or 28 days before challenge. When challenge occurred 7 days after treatment, fipronil/(S)-methoprene spot on was 100% effective against the tick infestation 48 hours after challenge and prevented infection with B. burgdorferi in all dogs. When the challenge was conducted 28 days after treatment, fipronil/(S)-methoprene spot on prevented all but 2 dogs from becoming infected with B. burgdorferi and was 97.6% effective against tick infestation 48 hours after challenge.
Introduction
Lyme disease has been reported in dogs throughout the United States, but occurs predominantly in northeastern states, mid-Atlantic states, upper midwestern states, and California. Infection occurs when the causative organism, Borrelia burgdorferi, is transmitted to a dog by the black-legged tick, Ixodes scapularis (also Ixodes pacificus).1 Morbidity attributable to borreliosis is commonly observed in endemic areas,2 suggesting a high prevalence of infection with clinical signs, most commonly limb or joint disease, developing in about 5% to 10% of infected dogs.
Avoidance of Lyme
disease in dogs is based on vaccination or prevention of I. scapularis
infestation, or at least limiting the duration of attachment to a
period less than required for transmission of B. burgdorferi. Although
transmission of the spirochetes has been reported as early as 37 hours
after tick attachment, these organisms were incapable of establishing
an infection. However, infection was established when organisms were
introduced 52 hours or more after tick attachment.3 Therefore, to
reduce the potential of transmission of B. burgdorferi, ticks must
be eliminated from the host within about 48 hours after attachment.
Thus, the time interval within which ticks die on animals treated
with acaricides is an important factor.3–5
Three formulations of fipronil, a 0.25% spray, a 10% spot-on, and a combination of 10% fipronil and 9% (S)-methoprene, an insect growth regulator, are commercially available for flea and tick control. Fipronil can be used on young dogs and has proven effective against a variety of ticks, including Rhipicephalus sanguineus (brown dog tick), Amblyomma americanum (lone star tick), Dermacentor variabilis (American dog tick), Ixodes scapularis (the black-legged tick), I. Ricinus, I. Holocyclus, and Haemaphysalis species as well as the cattle tick Boophilus microplus within 24 to 48 hours after challenge. For these species, at least 1 month efficacy is achieved after a single treatment.6 The purpose of these studies was to assess the efficacy of each formulation against a challenge performed 7 and 28 days after treatment with I. scapularis infected with B. burgdorferi and to determine whether efficacy against this challenge would prevent viable transmission of the B. burgdorferi organism to dogs.
Materials and Methods
Two parallel studies were conducted independently, using fipronil topical spray (0.25% w/v; FRONTLINE® Spray, Merial Limited, Duluth, GA, U.S.A.) or fipronil topical (10% w/v) combined with (S)-methoprene (9% w/v; FRONTLINE® Plus, Merial Limited, Duluth, GA, U.S.A).
Dogs
Each study included 24 purpose-bred beagle dogs, 12 females and 12 males. Dogs were acclimated to the testing facilities for 2 weeks and housed individually in stainless steel cages to prevent contact between them until after exposure to infected ticks was completed. After that, dogs were housed in runs until the end of the studies. All dogs were observed at least once daily for health problems. Both studies were approved and conducted in compliance with the TRS Labs, Inc., Institutional Animal Care and Use requirements, which regulates the use of animals in research.
For each study, dogs were allocated using a randomized block design in which eight replicates of three dogs each were formed based on descending body weights. Within replicates, dogs were randomly allocated to one of three treatment groups by lottery:
Group 1: Dogs in these groups were untreated controls.
Group 2: Dogs were treated with fipronil on Day –7 (7 days before infestation with I. scapularis).
Group 3: Dogs were treated with fipronil on Day –28 (28 days before infestation with I. scapularis).
Personnel involved with tick counts, serology, and tissue analysis were not aware of the treatment assignments.
Treatment Administration
In both studies, products were applied on Day –28 (28 days before tick infestation) for Groups 2 and on Day –7 (7 days before tick infestation) for Groups 3 (Table 1). Dogs in Group 1 remained as untreated controls.
In Study 1, fipronil topical spray was applied at the
label dose rate of 6 mL/kg to each dog. The dorsal, ventral and lateral
aspects of the torso, legs, and neck were sprayed, and the head was
treated by gently rubbing a sprayed gloved hand on the hair, avoiding
eyes and mouth.
In Study 2, topical fipronil combined with (S)-methoprene was applied as a spot-on formulation, directly onto the skin through parted hair, to the midline between the base of the skull and the shoulder blades, as per label directions. The label dose of 0.67 mL or 1.34 mL was applied for dogs within weight ranges of 11 to 22 and 23 to 44 lb, respectively.
Ticks, Borrelia burgdorferi, and Tick Exposure of Dogs
Adult black-legged
ticks, I. scapularis, were field-collected in the state of Rhode Island,
U.S.A. The rate of B. burgdorferi infection was 42%, based on a direct
fluorescence antibody assay of 30 randomly selected female ticks.
To establish tick infestations, dogs were placed in individual plastic basins after sedation by intramuscular injection of xylazine (1.1 mg/kg) and ketamine (10 mg/kg). Seventy-five ticks (approximately a 50:50 female to male ratio) were applied to the left shoulder of each recumbent dog. Ticks dispersed over the dog during approximately 1 hour of sedation.
Mapping and Counting Ticks on Dogs
Tick attachment sites were mapped and numbered by
marking the location of female ticks on paper
silhouettes assigned to each dog. The perimeter of attachment
sites was shaved to identify locations of possible future biopsies.
The process was repeated on 3 successive days after tick exposure.
Live ticks, attached and unattached, were counted approximately 48
hours after infestation by parting the hair and searching the entire
body. On Day 5 after tick exposure, the four sites of heaviest tick
attachment on each dog were identified for future biopsies and marked
on the dog’s silhouette; live ticks were also counted and removed
from each dog.
Serology
A
blood sample (7 mL) was collected from each dog on 8 occasions using
serum separator tubes (Table 1). Serum was recovered, aliquoted, and
stored at –20˚C until tested for antibody to B. burgdorferi by
a computerized kinetic enzyme-linked immunosorbent assay (KELA) as
described elsewhere.7 Diluted serum was added to duplicate wells in
microtiter plates containing antigens of French-pressed B. burgdorferi
soluble lysate. Bound antibody was detected by a goat anti-canine
antibody (heavy- and light-chain specificity) conjugated to horseradish
peroxidase (HRP, Cappel Research Products, Durham, NC, U.S.A.). Color
development, using the chromogen tetramethylbenzidine with H2O2 as a substrate,
was measured kinetically and expressed as the slope of the reaction
rate between enzyme and substrate solution. Each unit of slope was
designated as 1 KELA unit. A cutoff value of 100 KELA units was established
during prior validation of the assay to separate negative from positive
results; this cutoff value was confirmed by results of Western blotting.
Western blots were used for confirmation of infection in all dogs on Day 49 and on two dogs on Day 77 to clarify equivocal results of Day 49. Western blots were performed as previously described,8,9 with slight modification. The antigen preparation procedure was identical to that used for the KELA. Antigen was subjected to SDS-PAGE (12% acrylamide) at 200 V for 4 to 6 hours. Immunoreactive proteins were detected on nitrocellulose membranes using a miniblotter (Immunetics, Cambridge, MA, U.S.A.). Antibody was detected using a 1:10 dilution of serum followed by a conjugate consisting of goat anti-dog IgG conjugated to HRP (ICN Biomedical Research Products, Costa Mesa, CA, U.S.A). Western blots were considered positive if bound antibody was detected on at least three bands from among p39, p29–30, p28, p25–26, p22, and p19.
Culture and Polymerase Chain
Reaction Assay
Biopsies were collected (Table 1) from dogs under sedation with xylazine and ketamine as described previously. For each dog, a couplet of 3-mm skin punch biopsies was taken on day 77 or 78 from each of four multiple-tick attachment sites identified on Day 5 after tick exposure. If four sites of clustered ticks were not found on a given dog by Day 5, biopsies were taken from sites analogous to tick-cluster sites on control dogs. One sample of the biopsy couplet was destined for culture and the other for polymerase chain reaction (PCR) analysis. Briefly, the sites were shaved, thoroughly disinfected with Betadine® surgical scrub, rinsed with 70% alcohol followed by a saline rinse, and then dried with sterile surgical gauze. Single-use biopsy punches and disposable forceps were used to collect each sample. Samples were identified as recorded on Day 5 on silhouette maps of tick locations. Immediately after biopsy, samples were placed in 2- to 4-mL sterile cryovials and transferred to a cooler containing ice packs. They were shipped on dry ice the same day and arrived the next morning at the Diagnostic Laboratory, College of Veterinary Medicine, Cornell University, Ithaca, NY, U.S.A., where they were immediately processed and placed into culture or stored at –70˚C until subjected to PCR analysis.
For isolation of B. burgdorferi by culture, skin biopsies were homogenized in 0.5 mL of BSKII medium in a tissue homogenizer (Stomacher, Tekmor, Cincinnati, OH, U.S.A.) and then transferred into 6 mL of pre-warmed BSKII medium. Cultures were maintained at 34˚C and examined weekly for up to 6 weeks for evidence of B. burgdorferi growth using darkfield and indirect fluorescence antibody examination.
For PCR, the DNA from skin biopsy samples was extracted by a standard procedure.10,11 The DNA from B. burgdorferi was isolated, and PCR was performed as described using the SL primer set for the OspA gene (sense:5’-AATAGGTCTAATA ATAGCCTTAATAGC; antisense: 5’-CTAGTGTTTTGCATCTTCTTTGAAAA-3’). The primers were synthesized using a DNA synthesizer (Applied Biosystems 380A DNA Synthesizer, Foster City, CA, U.S.A.) at the Analytical and Synthetic Facility, Cornell University. To prevent contamination, the preparation of reaction mixtures, DNA extraction, amplification, and detection of PCR products were all performed in different rooms. Also, aerosol-resistant filter pipette tips were used throughout the experiment. Amplification of B. burgdorferi OspA was performed in a 50-?L reaction mixture of 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 0.5% NP40, 0.5% Tween 20, 200 mM of each of the deoxynucleoside triphosphates, 2 mM of primer sets (SL), and 2 U of the thermostable Taq DNA polymerase (Perkin-Elmer Cetus, Foster City, CA, U.S.A.) containing 100 ng of sample DNA. B. burgdorferi genomic DNA (1 ng) was used as a positive control and skin DNA samples from known B. burgdorferi negative dogs as the negative control. The reaction mixture was subjected to 40 cycles of amplification by using an automated DNA thermal cycler (Model 9600, Perkin-Elmer Cetus, Foster City, CA, U.S.A.). Each cycle involved heating to 94˚C for 1 minuted (DNA denaturation), cooling to 69˚C for 1 minute (primer annealing), and again heating to 72˚C for 2 minutes (primer extension). Negative controls were included in each PCR run, which consisted of a DNA template from a negative dog in the reaction mixture. Visualization of the PCR amplification products was performed by gel electrophoresis on a 1.5% agarose gel.
Statistical Methods
Tick counts were evaluated using Friedman’s test. Tick counts were transformed to the natural logarithm of (count + 1) for calculation of geometric means by treatment group.12 Efficacy was calculated using the Modified Abbott’s formula:
Efficacy (%) = (mc–mt) ¥ 100
mc
where: mc = mean No. ticks in control group
mt = mean No. ticks in treatment group
Fisher’s Exact test was used to determine the incidence of dogs testing positive in serology on KELA and Western blot, and in culture for the presence of B. burgdorferi. In all analyses, each treated group was compared to the appropriate control group.
Results
Efficacy Against I. scapularis
As presented in Table 2, all untreated control dogs in both studies were successfully infested with ticks (geometric mean of 22.4 and 24.5 ticks per dog in the spray and spot-on studies, respectively). For dogs treated 7 days before infestation, both formulations of fipronil were 100% effective.
For dogs infested 28 days after treatment, both the fipronil + (S)-methoprene topical spot-on and the fipronil spray formulations provided highly significant (P < 0.005) reductions of 97.6% and 99.6%, respectively. In the 28-day challenge for fipronil spray at 48 hours after exposure, 7 dogs were tick free and one harbored a single tick. In the spot-on study, 48-hour counts in dogs challenged 28 days after treatment showed 6 of the 8 dogs to be tick free, with the 2 other dogs carrying 3 and 8 ticks respectively.
B. burgdorferi Infection
Based on serology, none of the study dogs had been exposed to B. burgdorferi before initiation of the study. All 16 untreated dogs in both studies became infected with B. burgdorferi based on (a) seroconversion in all dogs; (b) positive cultures of biopsies (from all but 1 control dog in the spot-on study), and; (c) positive PCR results on skin biopsies (from all but one dog in the spot-on study). Of the 16 control dogs, 14 were positive in all biopsies collected, one was positive in 3 of the 4 biopsies, and the remaining dog was positive in 2 of the 4 biopsies.
None of the spray-treated dogs, challenged at 7 or 28 days after treatment and none of the spot-on-treated dogs challenged 7 days after treatment seroconverted at any time. All serology KELA values from these dogs were less than 100 at every collection (Figure 1A), indicating that there had been no effective transmission of the organism from the infected ticks. Culture results on all these dogs were uniformly negative and PCR results on all but one of the punch biopsy obtained on Day 77 of the study were negative, further confirming that treatment conferred protection from B. burgdorferi infection. From these groups, a single biopsy sample from one 7-day challenge spot-on-treated dog tested by PCR was positive; however, this individual dog was negative on both culture and serology. Six of 8 dogs treated with spot on 28 days before challenge remained serologically negative throughout the study. The other 2 dogs in this group, who remained infested with ticks 48 hours after challenge, developed antibodies to B. burgdorferi (Figure 1B), and skin biopsies from both were culture positive for B. burgdorferi. This was confirmed by PCR positive results in one of four biopsies, and two of four biopsies, respectively.
Discussion
In both studies, the proof of acaricidal efficacy with associated prevention of transmission of B. burgdorferi, depended on the certainty that B. burgdorferi infection was successfully introduced in untreated tick challenged dogs. Because of the possibility of false-negative results for culture and PCR, serology was added. All 16 control dogs (8 controls in each study) seroconverted, and 15 were PCR and culture positive. One dog remained culture and PCR negative but seroconverted with a high enzyme-linked immunosorbent assay (ELISA) titer that was confirmed positive by western blot. The negative PCR and culture result may be explained by the fact that organisms are not distributed homogeneously throughout the dog and could potentially be missed via punch biopsy. The data show that use of 3 assays to confirm infection heightened confidence that all dogs in the study were exposed to B. burgdorferi through the 75 ticks placed on each dog.
The interval within which tick mortality occurs on animals treated with acaricides is an important factor. In the fipronil spray study, one tick was found attached at 48 hours after tick exposure on one of the dogs that had been treated 28 days previously. This dog did not develop a B. burgdorferi infection. In the fipronil/(S)-methoprene spot-on study, two dogs treated 28 days before tick exposure had 3 and 8 ticks, respectively, still attached at 48 hours after exposure to ticks. Two days later, or approximately 96 hours after infestation, three ticks remained attached on one dog. These two dogs did seroconvert, indicating that ticks attached for more than 48 hours, but less than 96 hours, can successfully transmit the organism.
Given that B. burgdorferi infection rates in ticks within hyperendemic areas may vary from 30% to 70%, it follows that by reducing tick numbers attached to dogs by a range of 97.6% to 100% in fipronil-treated dogs, the risk of acquiring B. burgdorferi via tick bite is greatly reduced in treated dogs. Nevertheless, the fact that even one persistently attached tick can transmit infection suggests that treatment of dogs needs to be repeated through the tick season. Reduction of disease transmission can further be accomplished by appropriate vaccination in endemic areas.
Collectively, the data from this study shows that fipronil, in either the spot-on and spray formulation, is highly effective in preventing the transmission of B. burgdorferi to dogs by infected ticks. Choice of formulation, proper application procedures and maintenance of monthly compliance are facilitated greatly by client education in the veterinary hospital setting. When fipronil, used in a monthly basis, is coupled with other preventive measures, such as vaccination, education and tick avoidance strategies, the likelihood of dogs becoming infected by the Lyme agent is greatly reduced.
References
1. Dennis DT: Lyme disease. Dermatol Clin 13:537–551, 1995.
2. Levy SA, Magnarelli LA: Relationship between development of antibodies to Borrelia burgdorferi in dogs and subsequent development of limb/joint borreliosis. J Am Vet Med Assoc 200:344–347, 1992.
3. Straubinger RH, Straubinger AF, Jacobson RH, et al: Two lessons from the canine model of Lyme disease: Migration of Borrelia burdorferi in tissues and persistence after antibiotic treatment. J Spirochetal Tick-borne Dis 4:24, 1997.
4. Kelly C, Lake S, Mather T: Estimation of transmission probability of Lyme borreliosis. Biomet J 41:735–751, 1991.
5. Sood SK, Salzman MB, Johnson BJB, et al: Duration of tick attachment as a predictor of the risk of Lyme disease in an area in which Lyme disease is endemic. J Infect Dis 175:996–999, 1997.
6. Cruthers L, Slone RL, Guerrero J, et al: Evaluation of the speed of kill of fleas and ticks with Frontline® Top Spot® in dogs. Vet Therapeutics 2:170–174, 2001.
7. Appel MJG, Allen S, Jacobson RH, et al: Experimental Lyme disease in dogs produces arthritis and persistent infection. J Infect Dis 167:651–664, 1993.
8. Jacobson RH, Chang Y-F, Shin SJ: Lyme disease: Laboratory diagnosis of infected and vaccinated symptomatic dogs. Semin Vet Med Surg (Small Anim) 11:172–182, 1996.
9. Straubinger RK, Chang Y-F, Jacobson RH, et al: Sera from OspA-vaccinated dogs, but not those from tick-infected dogs, inhibit in vitro growth of Borrelia burgdorferi. J Clin Microbiol 33:2745–2751, 1995.
10. Chang Y-F, Novosel V, Chang C-F, et al: Experimental induction of chronic borreliosis in adult dogs exposed to Borrelia burgdorferi-infected ticks and treated with dexamethasone. Am J Vet Res 62:1104-1112, 2001.
11. Chang YF, Straubinger R, Jacobson RH, et al: Dissemination of Borrelia burdorferi after experimental infection in dogs. J Spiro Tick-Borne Dis 3:80–86, 1996.
12. Hollander M, Wolfe DA: Nonparametric Statistical
Methods. New York: John Wiley & Sons; 1973.
Table
1. Summary of Experimental Design for the Fipronil Spray and Fipronil
Plus (S)-Methoprene Trials
Day of Treatment Group
Experiment 1 2 3
–42 to –40 Pre-treat Ser Pre-treat Ser Pre-treat Ser
–28 Ser Rx & Ser Ser
–7 Ser Ser Rx & Ser
0 Ticks & Ser Ticks & Ser Ticks & Ser
0, 1, 2 Count and map ticks - all dogs
5 Count, map, and remove ticks from dogs
21, 35, 49, 63, 77 Ser Ser Ser
77-78 Biopsy Biopsy Biopsy
“Ser”
refers to bleeding of dogs for serology. “Rx” indicates treatments,
either of fipronil spray or fipronil plus (S)-methoprene. “Ticks”
refers to exposure of dogs to 75 ticks infected with B.
burgdorferi.
Table
2. Summary of Efficacy Against Ixodes Scapularis After Treatment With
Fipronil Spray and Fipronil Plus (S)-Methoprene Spot-on
Day 7 Day 28
Fipronil Spray 100% 99.6%
Fipronil + 100% 97.6%
(S)-Methoprene
spot-on
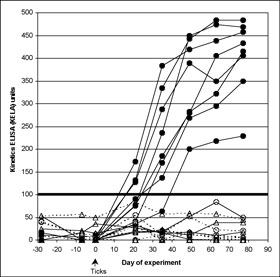
Figure 1. (A) Effect of fipronil spray on limiting tick-transmission of B. burgdorferi to dogs based on lack of seroconversion. Antibody levels to B. burgdorferi in untreated control Group 1 dogs (closed circles) and dogs treated with fipronil spray on Day -7 (open triangles; Group 3) or on Day –28 (open circles; Group 2). The cutoff separating positive from negative results was 100 KELA units. Eight Group 2, and eight Group 3 dogs are depicted but have overlapping symbols in the 0 to10 KELA unit range. All dogs were exposed to ticks infected with B. burgdorferi on Day 0 of the study.
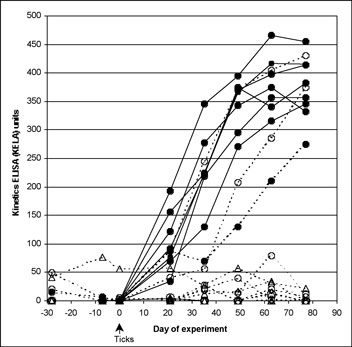
(B)
Effect of fipronil plus (S)-methoprene on limiting tick transmission
of B. burgdorferi to dogs based on lack of seroconversion. Antibody
levels to B. burgdorferi in untreated control Group 1 dogs (closed
circles) and dogs treated with fipronil plus (S)-methoprene on Day
-7 (open triangles; Group 3) or on Day –28 (open circles; Group 2).
The cutoff separating positive from negative results was 100 KELA
units. Eight Group 2 and eight Group 3 dogs are depicted but have
overlapping symbols in the 0 to10 KELA unit range. All dogs were exposed
to ticks infected with B. burgdorferi on Day 0 of the study.
ISSN# 1542-2666