|
The INTERNATIONAL JOURNAL of APPLIED RESEARCH In Veterinary Medicine |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The International Journal of Applied Research in Veterinary Medicine |
The Development of
a Rapid Diagnostic Test for Cortisol in the Saliva of Pigs
J.Lane, BSc, PhD
J. Flint, BSc
C.Danks, BSc
Central Science Laboratory, Sand Hutton, York, United Kingdom
This work was funded by the Animal Health and Welfare
Divison, DEFRA.
KEY WORDS: animal welfare, cortisol, lateral flow device, pig, saliva
Abstract
We have developed a rapid diagnostic kit for the assessment of salivary cortisol levels using lateral flow technology. These kits are similar to those used in home pregnancy tests consisting of a small plastic device containing a sample pad and a viewing window. Four drops of pig saliva are added to the sample pad, and after 5 minutes the result is read directly by looking for the presence of blue lines in the windows. The first line is a control to indicate that the test has been performed correctly and another blue line indicates that the level of cortisol is low. The absence of this second line denotes a high level of cortisol, indicating that the animal is stressed. The test is a competitive assay; therefore, unlike the conventional LFD format, a negative result is indicated by the presence of two lines and a positive detection by a single line. This kit will enable people with no scientific training to determine stress levels of pigs with no source of bias and ultimately, could provide a fast, cheap, disposable, and user-friendly method of assessing the stress levels of many different species of animals, including humans.
Introduction
With increased public awareness of animal welfare issues, the humaneness of farm animal management is coming under closer scrutiny. Live transport and differing housing regimens are of particular concern at present. Although many methods for assessing stress levels, and consequently welfare, exist, these tend to be invasive (blood sampling), impractical (collars for measuring heart rate), or time consuming (behavioral analyses). In addition, all these methods require scientifically trained personnel and often interfere, or are not consistent with, normal farming practices. An urgent requirement exists, therefore, to develop a simple, effective, and noninvasive way of assessing the welfare of these animals in the field.
Various hormones have been linked to the stress response. The most important of these are via the hypothalamic-pituitary axis (HPA) and the autonomic nervous system (ANS)1,2 (namely the corticoids). Hormone levels have traditionally been measured in the blood, involving some form of restraint and discomfort, and often causing changes in the physiologic parameters being measured. The use of noninvasive measures are becoming more widespread in the research fields as they have gradually gained acceptance as accurate ways of determining stress and therefore, poor welfare. Salivary cortisol as a measure of adrenal activity has been applied in humans since the early 1980s and is now seen as a reliable stress-marker.3-5 Direct correlations between salivary and plasma cortisol in domestic animals have been shown in basal and stressful situations.6,7 Saliva has the advantage of being relatively easy to collect, and its removal does not have any physiologic consequences.
Measuring hormones in body fluids, however, usually requires collecting samples and transporting them to a laboratory where assays such as an enzyme-linked immunosorbent assay (ELISA)8 or radioimmunoassay (RIA)9 are performed; normally taking in the region of several hours or more. There are obvious limitations to this practice. First, the time scale means that welfare problems cannot be immediately identified and rectified, and regular testing of a persistent problem would be difficult if not impossible. Second, these assays must be performed by trained scientific personnel using specialized equipment in a laboratory, which leads to high costs and inflexibility.
In medical circles, so-called "point-of-care" (POC) diagnostic testing is well established. This refers to tests that can be performed at the patient's bedside or in a doctor's surgery, allowing physicians to diagnose patients more rapidly than traditional laboratory-based testing. Many studies have shown that the rapid results that these POC tests produce can enable better therapeutic decisions, improved patient outcomes, and a reduction in the overall cost of care.10,11 Of all POC tests that have been developed, the immuno chromatographic or lateral flow devices (LFDs), have become the most established and accepted method.12,13 This area of diagnostics has grown dramatically in recent years, with the most common and well-known of these being the home pregnancy test.
Methodolgy
The Lateral Flow Device
The LFD is a small plastic device with a viewing window. Within the device are three sections: a sample pad, a reagent membrane, and an absorbent pad (Figure 1). The sample is added to the absorbent pad and then the device is left for a few minutes with the result read directly by eye, looking for the presence of blue lines. These kits are relatively cheap to make (in the region of a few U.K. pounds). They also have a long shelf-life and are fully disposable.
This technology is, therefore, ideally suited to field diagnostics. We have employed the LFD to determine the amount of cortisol in the saliva of a pig as a marker of its welfare.
The Mechanics of the LFD
The principle of the
competitive lateral flow assay relies on the competition for binding
sites on latex particles. Antibodies that are raised to a specific target,
in this case cortisol, are bound to dyed latex particles. These particles
are then applied using an immersion procedure onto a release pad, to
produce a stable particle reservoir for release onto a nitrocellulose-based
membrane. Two lines of reagent are immobilized onto the membrane using
a sophisticated reagent dispenser. The target reference or test line
comprises a conjugate of the target to be identified and the other,
the control, is a line of anti species antibody. The release pad and
membrane are assembled together with an absorbent pad into a plastic
housing, as illustrated in Figure 2.
Saliva droplets are added to the well, releasing the latex
particles, which then begin to flow across the membrane. If the target
antigen (cortisol) is present in the sample, antibody binding will occur
to produce a latex-antigen complex. Any latex particles that fail to
bind to antigen will attach to the immobilized test line as they traverse
the membrane; thus producing a visible line of deposited latex. The
anti species antibody on the control line captures any excess latex
particles, bound or unbound, to produce a control line as a visible
confirmation of latex flow. Sufficient target presence induces complete
inhibition of latex attachment to the test line, a result that is indicated
by a single line of latex attachment. The presence of two lines, therefore,
indicates a negative result.
Use of the LFD With Pig Saliva
The pig is allowed to chew on a large cotton bud until the bud is thoroughly moistened (about 30 to 60 seconds). Although centrifuging is the most common way to extract the saliva from the cotton bud,14,15 this would obviously not be possible in a farm situation. To ensure that all of this procedure is user-friendly, we needed to alter how the saliva was collected so that the sample could be easily gained in the field, with no specialized equipment or expertise required. This is achieved using a simple plunger system to extract the saliva from the swab. The saliva-soaked cotton wool is cut from the bud with scissors, placed under the plunger, and squeezed through the aperture of the tube (Figure 3). This collection technique is not only more suitable for a rapid diagnostic test in the field but has also resulted in a higher yield of sample with up to 1mL gained from a 15-kg pig (compared with approximately 300 ?L via centrifugation). This method also has the advantage of being more user-friendly, because the amount of sample needed for the LFD can be expressed as number of droplets rather than in microliters.
We found that saliva
runs very well across the membrane and can be used undiluted. The optimum
amount of saliva for the kits was found to be 70 mL (4 droplets),
which can be easily gained even from very small piglets.
After the saliva sample is taken from the pigs, the cotton bud is placed in the plunger system and the droplets are directly added onto the absorbent pad on the LFD. The device is left for 5 minutes, and the result read by looking for the presence of blue lines in the viewing window. The control line (showed in the picture by the C mark) indicates that the test is valid and the presence of the second line (indicated by the T mark) indicates a negative result (Figure 4).
Quantifying the LFD
Use of readers: This positive/negative lateral flow device can be used in a semi-quantifiable manner through the use of reader technology. A reader allows the intensity of the target line to be determined, facilitating the differentiation between target concentrations. Detailed analysis of lateral flow devices can be performed using a bench top reader attached to a PC (Figure 5A). Alternatively, there are easy to use and low-cost hand held readers available that enable target levels to be determined within seconds (Figure 5B). These palm-sized readers produce very sensitive and accurate results in a simple format that allow users to make quick on-site measurements of target levels.
Use of ladder LFD:
The alternative to using readers is to develop a lateral flow device,
which in itself is semi-quantitative. Research is currently underway
to produce a ladder lateral flow device that can detect the amount of
cortisol within a range of differing concentrations. The technology
used in the ladder device is identical to that of the standard LFD system.
However, rather than a simple positive/negative answer the ladder LFD
has multiple test lines (in the region of three to six) which aim to
give a result within a defined range. This will enable the user to determine
the amount of cortisol within a short range of concentrations (0-5,
5-10 ng/mL) (Figure 6).
Discussion
The development of POC tests and, in particular lateral flow devices, could have a major impact on the assessment of animal welfare. This kit has been developed to test whether the principle of lateral flow can be applied to the measurement of the stress hormone cortisol under normal farming conditions.
By using a rapid diagnostic test, welfare implications of farming procedures can be quickly and easily identified at a very low cost. Personnel assessing welfare with this method would need no scientific training, and, because no interpretation of the results is needed, bias and personal variation are eliminated. This enables it to be used by differently skilled people under varying conditions while retaining consistent results. The provision of this cheap and easy to use stress-marker will enable farmers and related personnel to incorporate this measure into the routine care of livestock.
Ultimately, these kits provide a rapid, economic, disposable and user-friendly method of assessing the stress levels of domestic animals under farming conditions.
Acknowledgments
The authors would like to thank Diane Owen, Sioban Ostoja-Starzewska and Matt Gomm for their assistance in all areas of this research.
References
1. H. Seyle: A syndrome produced by diverse nocuous agents. Nature 138:32-33, 1935.
2. Mostl E,
Palme R: Hormones as indicators of stress. Domestic Animal Endocrinol
23:67-74, 2002.
3. Guechot J, Fiet J, Passa P, et al: Physiological and pathophysiological variations in saliva cortisol. Hormone Res 61:357-364, 1982.
4. Hiramatsu R: Direct assay of cortisol in human-saliva by solid-phase radioimmunoassay and its clinical applications. Clinica Chimica Acta 117:239-249, 1981.
5. Aardal E, Holm A: Cortisol in saliva: Reference ranges and relation to cortisol in serum. Eur J Clin 33:927-932, 1985.
6. Parrot RF, Mission BH, Baldwin BA: Salivary cortisol in pigs following adrenocorticotrophic hormone stimulation: Comparison with plasma levels. Br Vet J 145:362-366, 1989.
7. Cook NJ, Schaefer AL, Lepage P, Morgan Jones S: Salivary vs serum cortisol for the assessment of adrenal activity in swine. Can J Animal Sci 76:329-335, 1996.
8. Cooper TR, Trunkfield AR, Zanella AJ, Booth WD: An enzyme-linked immunosorbent assay for cortisol in the saliva of man domestic farm animals. J Endocrin 123:R13-R16, 1989.
9. Cook NJ,
Schaefer AL, Lepage P, Morgan Jones SD: Radioimmunoassay for cortisol
in pig saliva and serum. J Agric Food Chem 45:395-3999, 1997.
10. Kiechle, Flingrammain R: Quality improvement and point-of-care testing. J Clin Ligand Assay 18:14-20, 1995.
11. Lee-Lewandrowski E, Lewandrowski K: Selected topics in point-of-care testing - Urinalysis, pregnancy testing, microbiology, fecal occult blood, and other tests. Clin Lab Med 21:389, 2001.
12. Bekkaoui F, Modrusan F, McNevin J, et al: CPT-based gene testing by a lateral flow device. Clin Chem 44:532-535, 1998.
13. Parsons M, Newman DJ, Pugia M, et al: Performance of a reagent strip device for quantitation of the urine albumin: creatinine ratio in a point of care setting. Clin Nephrol 51:220-227, 1999.
14. De Jong IC, Ekkel ED, Van de Burghal JA, et al: Effects of strawbedding on physiological responses to stressors and behaviour in growing pigs. Phys Behav 64:303-310, 1998.
15. Vining RF, McGinley RA, Maksyvitis JJ, Ho KY: Salivary cortisol: a better measure of adrenal function than serum cortisol. Ann Clin Biochem 20:329-335, 1983.
17. De Jong IC, Prelle IT, Van de Burghal JA, et al:
Effects of environment enrichment on behavioural responses to novelty,
learning and memory and the circadian rhythm in cortisol in growing
pigs. Phys Behav 68:571-578, 2000.

Figure 1. The
lateral flow device is shown.
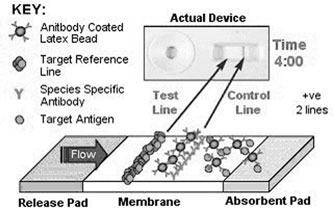
Figure 2. The mechanics of the lateral
flow device.

Figure 3. Plunger
system for the extraction of saliva from the cotton bud.

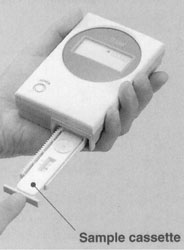
Figure
5. LFD Readers for quantifying
the LFD. (A) Benchtop reader which is attached to a personal computer.
(B) Hand-held reader is shown.

Figure 4. Lateral
flow device. The top device (A) shows the result gained when tested
on an unstressed pig. The device below (B) shows the result gained when
the levels of cortisol are high in the pig indicating stress and hence
poor welfare.
Figure 6. Ladder lateral flow device.
In this device there are 3 -6 test lines enabling a semi-quantitative
assessment of cortisol levels.
ISSN# 1542-2666